particle in a box approximation of metals The Particle in a Box (PIB) is a simple model that helps illustrate the behavior of electrons confined within atoms and molecules. It serves as a useful tool to introduce key quantum concepts: Energetic Quantization: Energy levels in .
How to successfully appeal a box junction penalty charge is not as easy as it sounds. You must provide solid proof with an appeal or a local authority will reject the appeal.
0 · particle in a box wikipedia
1 · particle in a box model
2 · particle in a box function
3 · particle in a box equation
4 · particle in a box diagram
5 · particle in a box
6 · formula for particle in a box
7 · 2 dimensional box particle formula
In search of an extra-large dog house that features solid construction and excellent features? Discover top XL dog houses and read our full guide for selecting a house for your large dog.
The particle-in-a-box eigenfunctions are given by Equation \(\ref{3.5.14}\), with \(B = 0\) and \(k = n\pi/L=a\), in accordance with Equation \(\ref{3.5.10}\) \[\psi _{n}(x)=A\, \sin\dfrac{n\pi x}{L} \label{3.5.14} \]
In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes the movement of a free particle in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example, a particle trapped inside a large box can move at any speed within the box and it is no more likely to be fo.
A particle in a 2-dimensional box is a fundamental quantum mechanical approximation describing the translational motion of a single particle confined inside an infinitely deep well from which it .The particle in a box model lets us consider a simple version of the Schrödinger equation. Before we simplify, let's take another look at the full Hamiltonian for a particle-wave in three dimensions (see equation 2.2.2) and the simplest form .Explain why the energy of a quantum particle in a box is quantized. Describe the physical meaning of stationary solutions to Schrӧdinger’s equation and the connection of these solutions with time-dependent quantum states. Explain .The Particle in a Box (PIB) is a simple model that helps illustrate the behavior of electrons confined within atoms and molecules. It serves as a useful tool to introduce key quantum concepts: Energetic Quantization: Energy levels in .
particle in a box wikipedia
• Particle in a box approximation – you solve the Schrödinger equation. Practice Questions 1. The energy levels of the particle in a box are given by εn = ℏ2n2p2/2 mL 2. (a) Why does the .
Approximately, therefore the particle in a box in a finite potential energy well can be considered as a first (crude) approximation model of an atom. At least for the purpose of demonstrating the . We will show how this relationship can be derived from the results of the 1D particle in a box. Additionally, we will show how the particle in a box model can be applied to make .
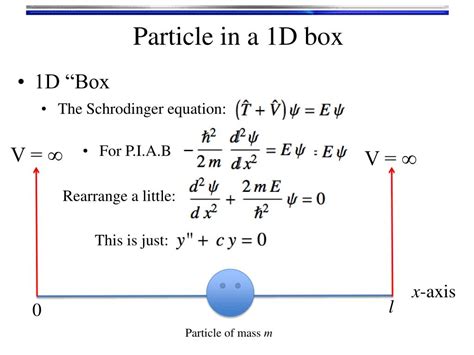
Free particle and the particle in a box. Schrödinger equation is a 2nd-order diff. eq. 2 ∂2ψ ( x ) − + V ( x )ψ ( x Eψ ( x. ) 2m ∂x2. We can find two independent solutions φ. ( x ) and φ. CONCEPT:. Energy Levels in a 1D Particle-in-a-Box System and Variational Method. For a particle confined in a 1D box (potential energy ( V = 0 ) inside the box and ( \(V = \infty \)) outside), the energy levels are quantized, with the ground state energy denoted as ( E 0). The expectation value of the Hamiltonian with a trial wave function provides an estimate of the .
particle in a box model
Step 1: Define the Potential Energy V. The potential energy is 0 inside the box (V=0 for 0L). We assume the walls have infinite potential energy to ensure that the particle .
Why in the particle in a box model do the values of n begin at 1 but in the harmonic oscillator they begin at 0? I understand what the wave-functions and their corresponding probabilities look like and that the PIB has 0 nodes for n=1 which means the number of nodes is n-1, so for n = 0 it would have -1 nodes which is physically unreasonable.Free particle and the particle in a box . The energy spacings increase as the box size decreases. 1 E ∝ a2. 5.61 Fall 2007 Lecture #7 page 5 We’ve solved some simple quantum mechanics problems! The P-I-B model is a good approximation for . The Particle-In-A-Box approximation. Electrons in the \( \pi \)-electron system of a conjugated aromatic compound are not restricted to specific nuclei but are free to move throughout the system. In a linear conjugated system the potential energy of the electrons will vary along the chain, being lowest near the nuclei and highest between them.Figure \(\PageIndex{2}\): Visualizing the first six wavefunctions and associated probability densities for a particle in a two-dimensional square box (\(L_x=L_y=L\)).Use the slide bar to independently change either \(n_x\) or \(n_y\) quantum number and see the changing wavefunction. Unlike in the one-dimensional analoge, where nodes in the wavefunction are .
particle in a box function
It states that we cannot know both the position and momemtum of a quantum particle with complete certainty. We will show how this relationship can be derived from the results of the 1D particle in a box. Additionally, we will show how the particle in a box model can be applied to make predictions on real systems. 4.5.5.2. Learning Goals:#Classical vs Quantum particle in a box#. Particle in a box is a toy model of electron (or atom, molecule, small quantum object) trapped in some region of space \([0,L]\).. The positional information of a quantum “particle” is described by a quantum wave function \(\psi(x)\) which is obtained by solving Schrödinger equation with boundary conditions .
9 Particle-in-a-box(PIB) 1. Consider a linear poly-ene. 2. Theelectrons are completelydelocalized insidethepoly-ene, butcannotleavethe molecular framework. 3. Let us approximate this system by a one-dimensional box, of length L. The potential energy of the electrons inside the polyenes can be approximated by the figure below. 4.The conventional quantum mechanical problem of a particle in a one-dimensional box can be made more interesting chemically without introducing any more difficult mathematics by employing a potential diagram rather than the usual infinite well.
Answer to 17. The molecular orbital energies of butadiene. 17. The molecular orbital energies of butadiene CH2=CH-CH=CH2 can be ap- proximately represented either using the particle-in-a-box model or using the Hückel approximation (although the latter approximation significantly under- estimates the energies of anti-bonding molecular orbitals).
The particle-in-a-box model for motion in one or two dimensions discussed earlier can obviously be extended to three dimensions. . This same spherical box model has also been used to describe the valence electrons in quasi-spherical nano-clusters of metal atoms such as \(Cs_n\), \(Cu_n\), \(Na_n\), \(Au_n\), \(Ag_n\), and their positive and .and sketch this function: the particle is more likely to be found in the left-hand half of the box. Now, suppose the time is t = 4 m L 2 / h, so e − i π h t 4 m L 2 = − 1. At this time, ψ (x, 2 L 2 / h) = 1 L (− sin π x L + sin 2 π x L) and it’s easy to see that .An electron moving in a thin metal wire is a reasonable approximation of a particle in a one-dimensional infinite well. The potential inside the wire is constant on average but rises at each end. Suppose the electron is in a wire 1.0 cm long. (a) Compute the ground-state energy for the electron. (b) If the electron If you consider your wave-function, with sinusoidal changes within a box, the particle is considered a wave with energies in a probable range. So of course the attributes of a wave will change given the length of your box. The choice of boundary condition affects the physical probabilities.

An electron moving in a thin metal wire is a reasonable approximation of a particle in a one-dimensional infinite well. The potential inside the wire is constant on average but rises sharply at each end. Suppose the electron is in a wire 1.0 cm long. 1) Find the ground-state energy for the electron.Answer to Based on the particle in a one-dimensional box. Science; Chemistry; Chemistry questions and answers; Based on the particle in a one-dimensional box approximation for polyenes, suggest where along the line segment the n=1 to n=2 electronic transiton would most likely take place. explain your choiceAlthough a particle in such potential is an idealization, it is a very important problem because: a) Exact solutions are obtained from the Schrodinger Equation. b) It demonstrates important features of quantum-mechanical problems. c) This potential is a good approximation to some real situations such as a free electron in a metal. Question: To a crude first approximation a pi electron in a linear polyene may be considered as a particle in a 1D box. To a crude first approximation a pi electron in a linear polyene may be considered as a particle in a 1 D box. Here’s the best way to solve it. Solution.
particle in a box equation
The quantum particle in the 1D box problem can be expanded to consider a particle within a higher dimensions as demonstrated elsewhere for a quantum particle in a 2D box.Here we continue the expansion into a particle trapped in a 3D box with three lengths \(L_x\), \(L_y\), and \(L_z\). As with the other systems, there is NO FORCE (i.e., no potential) acting on .where the momentum = ℏ and the numbers n x, n y, and n z are any set of integers– positive, negative, or zero. We can imagine setting up coordinate axes k x, k y, and k z and putting a point everywhere in k-space that there is an allowed state. Notice that as the size of the box gets bigger, the states get closer together in k-space. Since E = ℏ 2 k 2 ∕ 2 m, the lower energy .
We will introduce the Drude model, a reasonable description of electrons in metals. The quasi-static approximation will then let us take into account the nanoscale size of the particles. Additionally, the generalization of the Clausius–Mossotti relation will let us consider a particle embedded in a dielectric medium and the effect of its .
The density of states related to volume V and N countable energy levels is defined as: = = (()). Because the smallest allowed change of momentum for a particle in a box of dimension and length is () = (/), the volume-related density of states for continuous energy levels is obtained in the limit as ():= (()), Here, is the spatial dimension of the considered system and the wave vector. The particle in a box is the very first example most people see of a bound state problem. These are a class of quantum mechanical problems whereby we see, by simple mathematics, that the energy levels of certain quantum systems are discretely quantized. . Using Sterling's approximation, $\log{N!}\sim N\log{N}-N$, we arrive atWe now turn our attention to a generalization of the 1-dimensional quantum box to 3 dimensions. The 3 - dimensional quantum box is shown schematically in figure 1.5. It extends along 0 ^ x ^ L x, 0 ^ у ^ L y, 0 ^ z ^ L z. Outside this region the potential is infinite so that the wavefunction ¥ is zero at the faces of the box.
Particle in a box with finite-potential walls# 2.6.1. . Consider the case the situation of electrons in a metal. We saw when looking at the Photoelectric effect that a reasonable approximation of the potential that confines the electrons within the metal had a finite depth. This is the potential we will now consider and it is be far the most . As with Example 7.4.1 , we recognize that unperturbed component of the problem (Equation \(\ref{7.4.2}\)) is the particle in an infinitely high well. For this system, the unperturbed Hamiltonian and solution is the particle in an infinitely high box and the perturbation is a shift of the potential within half a box by \(V_o\).
particle in a box diagram
particle in a box
Yellow box junctions are designed to prevent a road becoming blocked and keep traffic flowing. However, they can also catch drivers unawares, resulting in a fine of up to £130. Many are.
particle in a box approximation of metals|particle in a box wikipedia